The research efforts of my laboratory have focused on understanding how cellular signaling pathways influence cell growth and differentiation, as well as how aberrant signaling activities lead to disease states such as cancer and other disorders. The laboratory is multi-disciplinary using various cell systems and mouse models to identify novel signaling events that influence fundamentally important biological processes, and chemistry and structural approaches to define the molecular basis for these events. Four complimentary areas of research are currently being pursued in the laboratory.
1) Studies of the regulation and structural characterization of the Ras-related small G protein, Cdc42, and related Rho GTPases.
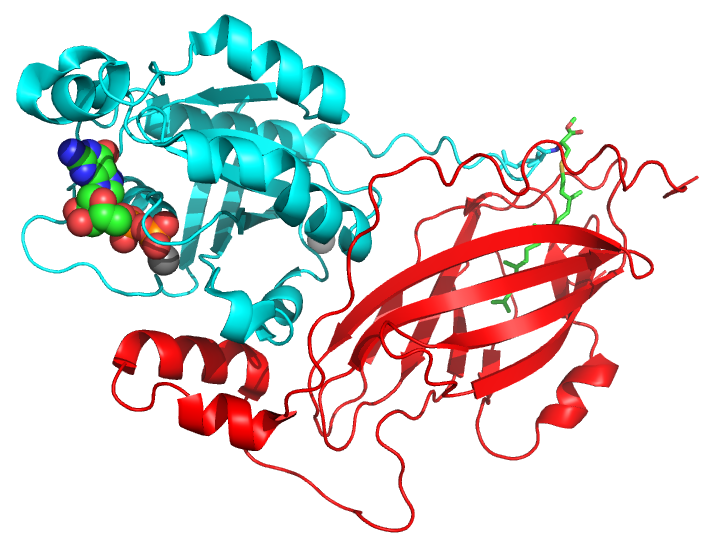
These G proteins and their regulators play critical roles in cell growth, the establishment of cell polarity, and cytokinesis. We have been particularly interested in examining the roles of Cdc42 and its downstream signaling partners in mammalian development, and in particular, in neurogenesis. Below is a crystal structure of Cdc42 bound to its regulator, RhoGDI.
PDB: 1DOA
2) Delineating the signals that impact the metabolic machinery of cancer cells and virus-infected cells.
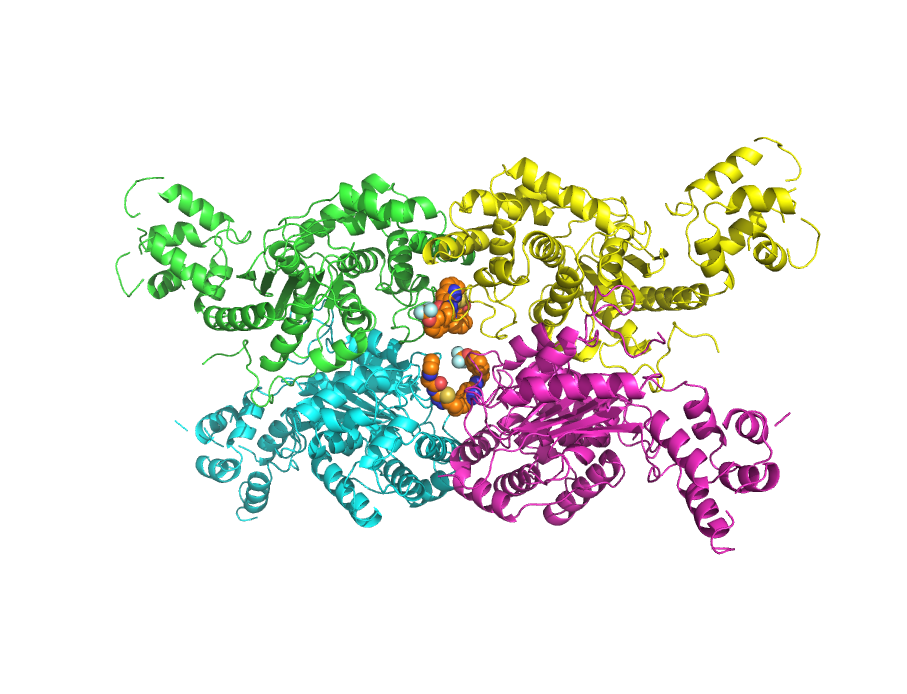
We have been focusing on understanding the signaling regulation of key metabolic enzymes that are responsible for the “glutamine addiction” exhibited by cancer cells as well as virus-infected cells undergoing the “Warburg effect”, and in the development of small molecule inhibitors targeting these enzymes as drug candidates. Below is a crystal structure of glutaminase bound to an allosteric inhibitor, CB-839.
PDB: 5HL1
3) Structure-function studies of G protein-coupled receptors, their G protein partners, and other interesting signaling proteins.
We have used the sensory response system responsible for vertebrate vision to obtain structural pictures for how a G protein-coupled receptor, the photoreceptor rhodopsin, activates its G protein signaling partner, transducin, and how activated transducin stimulates its biological effector, the cyclic GMP phosphodiesterase to mediate phototransduction. We are combining fluorescence spectroscopic approaches with X-ray crystallography (both traditional methods and newly emerging room temperature crystallography), small angle X-ray scattering, and cryo-electron microscopy to analyze the three-dimensional structures of rhodopsin bound to wild-type transducin and different novel transducin mutants, as well as the entire phototransduction complex of rhodopsin, transducin, and the cyclic GMP phosphodiesterase. We also have been performing structure-function studies of a GTP-binding (G protein)/protein crosslinking enzyme (TGase-2) that has been implicated in promoting aggressive cancers with the goal of designing new classes of drug candidates targeting this protein.
4) The biochemistry and clinical relevance of extracellular shed vesicles (microvesicles and exosomes).
These vesicles, which are generated in response to Rho GTPase-signaling and different extracellular cues, contribute to a number of different aspects of biology, including aging, neurobiology, stem cell biology, cancer, and virus infection. In the case of cancer, they exert important influences on the tumor microenvironment, the creation of a pre-metastatic niche at secondary sites of tumor colonization, tumor angiogenesis and the evasion of an immune response. We are trying to determine how these vesicles are loaded with specific protein and RNA cargo, how they contribute to different biological and disease processes and whether we can regulate their production as a means to provide new strategies of therapeutic intervention.